This guide offers a deep dive into the intriguing chemical formula hcooch ch2 h2o, providing clear explanations, detailed analysis, and expert insights. Whether you are a researcher, a student, or simply curious about the compound, this article is designed to equip you with a comprehensive understanding of its various interpretations, synthesis methods, properties, and applications.
The chemical string hcooch ch2 h2o has sparked considerable discussion due to its ambiguous presentation. At first glance, it might be interpreted in several ways, leading to debates about its true molecular identity. In this guide, we aim to clarify these interpretations and provide detailed explanations regarding the structure, properties, synthesis techniques, and real-world applications of hcooch ch2 h2o.
This article is intended for a broad audience. Beginners will gain fundamental insights, while advanced readers will appreciate the in-depth discussion of chemical properties and synthesis methods. By covering every aspect of the topic, we strive to create the definitive resource that not only informs but also engages readers with clear examples, tables, and practical lists.
Decoding the Formula: hcooch ch2 h2o
Understanding hcooch ch2 h2o begins with breaking down the notation. The formula appears to incorporate fragments such as “HCOO,” “CH₂,” and “H₂O.” However, its presentation leaves room for multiple interpretations. Let’s explore these interpretations in detail.
Possible Interpretations
One common interpretation of hcooch ch2 h2o is that it might be a misformatted representation of a known compound. Two prominent possibilities include:
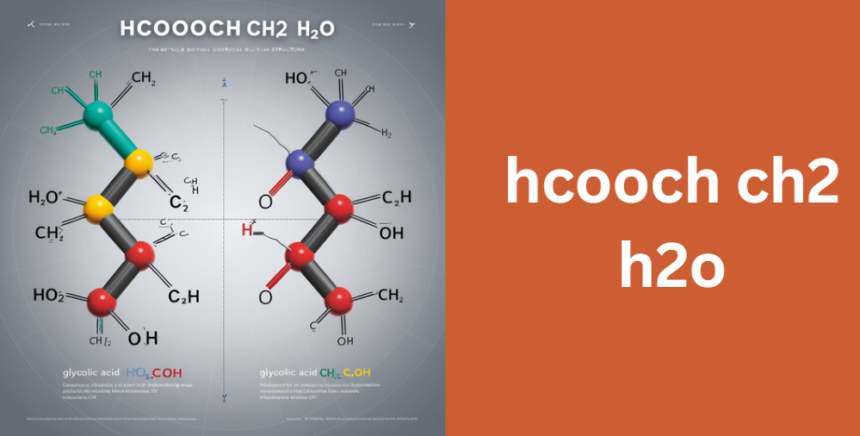
- Glycolic Acid (HOCH₂COOH):
This interpretation considers the formula as an abbreviation or a typographical error. Glycolic acid, which has the formula HOCH₂COOH, is widely recognized in both industrial and laboratory settings. Its simple structure and extensive applications in skincare and chemical synthesis make it a prime candidate. - Alternative Ester Form (HCOOCH₂OH):
Another perspective is that the formula could represent an ester form, specifically HCOOCH₂OH. In this case, the compound is a structural isomer of glycolic acid, meaning that while it shares the same molecular formula (C₂H₄O₃), its connectivity differs, resulting in unique chemical properties.
Comparative Analysis
To better understand the differences between these interpretations, consider the following table:
| Aspect | Glycolic Acid (HOCH₂COOH) | Alternative Ester (HCOOCH₂OH) |
|---|---|---|
| Molecular Formula | C₂H₄O₃ | C₂H₄O₃ |
| Structure | Carboxylic acid group | Ester linkage |
| Common Uses | Skincare, organic synthesis | Less common, specialized research |
| Physical Properties | Well-documented properties | Less characterized properties |
This table highlights the similarities and differences in structure and application, reinforcing the need for precise context when discussing hcooch ch2 h2o.
Historical Background and Nomenclature
Understanding the evolution of the compound’s identification helps place hcooch ch2 h2o in context. Over the years, researchers have debated its precise structure, leading to various naming conventions.
Evolution of Identification
Historically, compounds like these have been described differently based on emerging analytical techniques. Early researchers relied on basic chemical reactions and simple spectroscopic data, which sometimes resulted in multiple interpretations of the same formula.
Nomenclature Standards
Modern chemical nomenclature follows IUPAC guidelines, which help standardize naming. For instance:
- Glycolic acid is systematically named HOCH₂COOH.
- The ester form might simply be referred to by its systematic name or as an isomer of glycolic acid.
Understanding these distinctions is crucial for accurately interpreting research literature and experimental results involving hcooch ch2 h2o.
Molecular Structure and Chemical Properties
A thorough exploration of the molecular structure and chemical properties of hcooch ch2 h2o is essential for both theoretical studies and practical applications.
Detailed Molecular Structure
The molecular structure can be visualized using both 2D Lewis structures and 3D models. Consider the following simplified depiction:
| Component | Representation | Role in Structure |
|---|---|---|
| HCOO | Formate group | Contributes to reactivity as an acid or ester component |
| CH₂ | Methylene group | Acts as a linker between functional groups |
| H₂O | Water molecule | Could indicate hydration or a side component in the synthesis |
These components, when arranged correctly, form either glycolic acid or its isomer, with each arrangement imparting different chemical behaviors.
Physical and Chemical Properties
The physical properties such as boiling point, melting point, and solubility vary depending on the structure:
- Spectroscopic Signatures: Infrared (IR) spectroscopy, Nuclear Magnetic Resonance (NMR), and Mass Spectrometry (MS) provide fingerprints for each interpretation of hcooch ch2 h2o.
- Reactivity: The acidic or ester functionalities influence how the compound reacts in chemical processes, including potential isomerization and equilibrium reactions.
Synthesis and Production Methods
Creating hcooch ch2 h2o in a laboratory or industrial setting requires careful planning and the use of precise chemical reactions.
Laboratory Synthesis Techniques
Researchers have developed step-by-step synthetic routes that may include:
- Reactant Preparation: Starting with known chemicals such as formaldehyde or formic acid.
- Reaction Conditions: Controlling temperature, pH, and catalysts to steer the reaction toward the desired structure.
- Purification: Utilizing techniques like distillation or chromatography to isolate the target compound.
A diagram illustrating the synthesis pathway could be as follows:
[Formic Acid] + [Formaldehyde] --(Catalyst, Temperature)--> **hcooch ch2 h2o** (as glycolic acid or ester form)
Industrial Production and Scale-Up
When scaling up the synthesis:
- Consistent quality control is critical.
- Advanced purification methods ensure that the final product meets industry standards.
Analytical Techniques
Verification of the compound is carried out using:
- Chromatography: For separating components.
- Spectroscopy: For confirming the molecular structure.
- Computational Validation: Software tools simulate molecular behavior and predict reactivity.
Applications and Uses
Understanding the applications of hcooch ch2 h2o reveals its importance across various fields.
Organic Synthesis and Chemical Manufacturing
The compound often serves as an intermediate in more complex organic reactions. Its functionality enables:
- The production of polymers and advanced materials.
- Use in organic reactions that require either an acidic or ester functional group.
Pharmaceutical and Biomedical Applications
In the biomedical realm, compounds related to hcooch ch2 h2o show promise for:
- Therapeutic applications where specific reactivity is desired.
- Use as a building block in drug synthesis, where its unique properties can be harnessed for medicinal chemistry.
Environmental and Industrial Impact
Advances in green chemistry have led to more sustainable synthesis methods. Environmental benefits include:
- Reduced use of hazardous chemicals.
- More efficient production processes that minimize waste.
A brief table summarizing the applications is presented below:
| Field | Application | Benefit |
|---|---|---|
| Organic Synthesis | Intermediate in complex reactions | Enhances reaction efficiency |
| Pharmaceuticals | Drug development and formulation | Provides unique functional groups |
| Green Chemistry | Eco-friendly synthesis techniques | Promotes sustainability and safety |
Analytical Methods and Research Tools
High-quality research on hcooch ch2 h2o depends on advanced analytical methods.
Spectroscopic and Chromatographic Analysis
State-of-the-art techniques include:
- IR Spectroscopy: Identifies functional groups.
- NMR Spectroscopy: Provides detailed structural information.
- Mass Spectrometry: Confirms the molecular weight and composition.
Computational Chemistry and Modeling
Modern research leverages computational tools to simulate molecular behavior. Software such as Gaussian or Spartan aids in:
- Predicting reaction pathways.
- Validating experimental results through theoretical calculations.
Recent Experimental Advances
Emerging methods continue to refine our understanding of hcooch ch2 h2o. Breakthroughs in nano-scale synthesis and enhanced imaging techniques are paving the way for new applications and improved synthesis routes.
Safety, Handling, and Environmental Considerations
When working with chemicals like hcooch ch2 h2o, safety and environmental impact are paramount.
Handling Protocols
Laboratories must adhere to strict safety guidelines:
- Refer to Material Safety Data Sheets (MSDS) for proper handling.
- Use personal protective equipment (PPE) such as gloves, goggles, and lab coats.
Environmental Impact
Green chemistry initiatives aim to reduce hazardous waste and improve energy efficiency. Sustainable practices include:
- Recycling solvents and reagents.
- Utilizing less toxic catalysts.
Regulatory Standards
Compliance with local and international regulations is crucial. Regular audits and certifications help ensure that research and industrial processes meet stringent safety standards.
Current Trends and Future Directions in Research
Research into hcooch ch2 h2o is dynamic and continually evolving. Recent scientific discoveries and technological advances are pushing the boundaries of what we know about this compound.
Recent Scientific Discoveries
Several breakthrough studies have shed light on the unique properties of hcooch ch2 h2o. Research published in leading journals has:
- Clarified the structural nuances of the compound.
- Demonstrated novel synthesis routes that improve yield and purity.
Emerging Applications
New applications are emerging in areas such as:
- Advanced materials science, where hcooch ch2 h2o may be used in nanotechnology.
- Biotechnology, where its specific reactivity could aid in the development of innovative therapies.
Research Gaps and Future Challenges
Despite these advances, significant challenges remain:
- A need for standardized methodologies to distinguish between isomers.
- More detailed studies on long-term environmental impact and industrial scalability.
A concise list of future research opportunities includes:
- Investigating alternative synthesis routes.
- Exploring computational models to predict reactivity.
- Conducting interdisciplinary studies to integrate findings across fields.
Frequently Asked Questions (FAQs)
What is the primary interpretation of the formula hcooch ch2 h2o?
The most common interpretation is that it may represent glycolic acid (HOCH₂COOH), though alternative ester forms such as HCOOCH₂OH are also considered.
How can one synthesize hcooch ch2 h2o in a laboratory?
Standard synthetic routes involve combining precursors like formic acid and formaldehyde under controlled conditions, followed by purification using chromatography and spectroscopic verification.
What are the applications of hcooch ch2 h2o in industry?
The compound is used as an intermediate in organic synthesis, a building block in pharmaceutical production, and has potential applications in green chemistry for sustainable processes.
Conclusion
In summary, our guide to hcooch ch2 h2o has provided a thorough examination of its structure, synthesis methods, applications, and future research directions. By exploring multiple interpretations and detailing both laboratory and industrial perspectives, we hope to have clarified the complexities surrounding this compound. Whether you are engaged in academic research or industrial applications, understanding the nuances of hcooch ch2 h2o is essential for leveraging its full potential.
This article serves as a comprehensive resource designed to inform, engage, and empower readers with all the necessary information to excel in their research or applications related to hcooch ch2 h2o.
Other Posts Like hcooch ch2 h2o
The Definitive Guide to the 6muva90 needle: Specifications, Applications, and Best Practices
myrkgard stronghold walls came tumbling down]: Complete Info
Who is baeily_irishcr3m3? The Ultimate Guide to Her Life, Career & Online Influence